Abstract
Background:
Allogeneic stem cell transplantation (HCT) is a potentially curative therapy for patients with myelofibrosis (MF). Factors associated with survival in the literature include donor source, age, conditioning regimen, and spleen size prior to transplant. We reviewed the transplant outcomes from Mayo Clinic from the last 10 years to identify pre-transplant factors associated with survival.
Methods:
Patients undergoing HCT for MF at the 3 sites of Mayo Clinic in Rochester, Arizona and Florida, between January 2005 and August 2016, were retrospectively reviewed. Survival analysis was conducted using Kaplan-Meier (K-M) analysis and a series of univariable and multivariable Cox proportional hazard regression models. Patient characteristics measured before bone marrow transplant procedure along with patient's age, gender, transfusion dependence, and Dynamic International Prognostic Scoring System (DIPSS)-Plus score, and transplant characteristics including conditioning regimen (Reduced intensity conditioning (RIC) vs myeloablative conditioning (MAC)), donor (related vs unrelated), and graft versus host disease (GVHD) prophylaxis (Calcineurin inhibitor (CNI) +methotrexate (MTX) or CNI + mycophenolate mofetil (MMF)), and number of anti-thymocyte globulin (ATG)doses administered (dose 2.5 mg/kg; grouped as 0 doses, 1 dose, or 2-3 doses) were used to examine differences in K-M curves and to predict overall survival in the regression models.
Results:
There were 87 patients included in this analysis. The median age at HCT was 58 (19-73), and there was a male predominance (n=52, 60%). Sixty patients (69%) had primary MF, and the remainder had secondary MF. The DIPSS plus score was high risk in 37 (43%), Intermediate-2 risk in 45 (52%), and Intermediate-1 risk in 5 (6%) patients. JAK2 mutation was present in 41(47%). A RIC regimen was used in 73 (84%). ATG was given in 37 patients (43%). The median time to platelet and neutrophil engraftment were 23 days (8-116) and 17 (10-166) respectively. There were 13 patients who never engrafted platelets, and 3 patients who never engrafted neutrophils. At the time of last follow up, 53 (61%) patients were alive. Relapse occurred in 12 (14%) patients. With a median of 37.2 (5-629) months follow up, the overall probability of survival was 66% (95% CI 57-77%) at 2 years, and 57% (95% CI 46-70%) at 5 years. Treatment related mortality occurred in 25 patients (29%), relapse related mortality was found in 8 (9%). Grade 2-4 acute GVHD occurred in 49 (56%) and grade 3-4 GVHD occurred in 28 (32%). Chronic GVHD occurred in 33 (45%), 7 (21%) were mild, 19 (58%) were moderate, and 7 (21%) were severe.
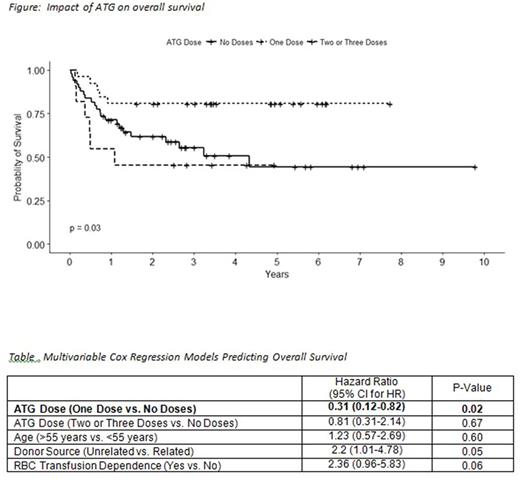
Of the characteristics considered, ATG dose, donor source, and RBC transfusion dependency showed a significant difference in survival between groups (p < .05) (See Figure). In the multivariable analysis, (See table) one dose of ATG as compared to no doses was significantly associated with increased survival [HR = 0.31 (0.12-0.82), p < .05]; unrelated donor source [HR = 2.2 (1.01-4.78), p= .05] and RBC transfusion dependence [HR=2.36 (0.96-5.83), p= 0.06] were associated with an increased risk of death but these relationships were not statistically significant. This model was adjusted for age, but age was not significantly related to increased risk of death [HR = 1.23 (0.57-2.69), p= .60].
Discussion:
In our retrospective analysis of patients undergoing HCT for MF, there were several notable findings. Receipt of low dose ATG was associated with a favorable survival, whereas, need for RBC transfusion pre-HCT and unrelated donor were associated with a trend towards increased risk of death. This association of low dose ATG use and improved survival as seen in our study would need to be studied in larger prospective study to further elucidate the reasons and validate of these findings.
Khera: Novartis: Consultancy. Mesa: Incyte Corporation: Research Funding; CTI BioPharma Corp.: Research Funding; Gilead Sciences, Inc.: Research Funding; Galena Biopharma, Inc.: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy; Celgene Corporation: Research Funding; Ariad: Consultancy; Promedico: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal